7. The specific heat capacity of a metal block of mass m is determined by placing a heating coil in its centre, as shown in the diagram above.
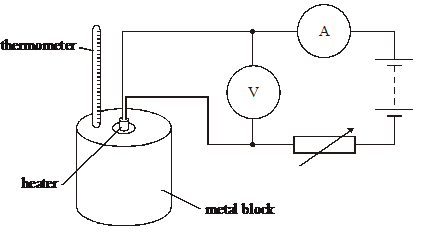
The block is heated for time t and the maximum temperature change recorded is Äè. The ammeter and voltmeter readings during the heating are I and V respectively.Which one of the following is not a source of error in the experiment?